Edited by Dr. Marie Besnier, Head of Life Science at Inventia Life Science
Review recent advancements in in vitro fibrosis models and their significance in research and drug development.
TABLE OF CONTENTS
Understanding Fibrosis and its Impact on Health
The Importance of In Vitro Models in Fibrosis Research
Advancements in In Vitro Fibrosis Models
Applications of In Vitro Fibrosis Models in Drug Development
Challenges and Future Directions in Fibrosis Research
Additional Articles to Explore
UNDERSTANDING FIBROSIS AND ITS IMPACT ON HEALTH
Fibrosis is characterized by an excessive accumulation of scar tissue in various organs, including the liver, lungs, and kidneys. While it can occur naturally with aging, it is a common feature of various diseases, such as liver cirrhosis and tissue cancers, and significantly impacts an individual’s health and quality of life.
The development of fibrosis is a complex process involving multiple molecular mechanisms and pathways. It typically starts with an injury that leads to inflammation, which in turn triggers the activation of fibroblasts, the principal cells responsible for producing collagen and other extracellular matrix proteins. Over time, the excessive production and deposition of matrix proteins lead to the formation of scar tissue that impairs the normal functioning of the affected organ.
While fibrosis was once thought to be irreversible, recent studies suggest that it could be reversed in particular conditions. Therefore, designing effective therapeutic strategies requires an understanding of the underlying mechanisms of fibrosis progression.
THE IMPORTANCE OF IN VITRO MODELS IN FIBROSIS RESEARCH
In vitro models of fibrosis involve the culture of fibroblasts and other tissue-relevant cell types in the laboratory to provide a simplified representation of in vivo conditions. Such models can be used to conduct controlled experiments that manipulate and isolate specific variables involved in fibrosis development while accounting for confounding factors. By altering various parameters of the in vitro model, such as cell types, extracellular matrix composition, stiffness, and biochemical signaling pathways, researchers can interrogate the underlying processes that drive fibrosis progression.
The knowledge gained from research using in vitro models drives the identification of potential therapeutic targets, which facilitates the development of new drugs to treat fibrosis. Given the complexity and diversity of fibrosis development, as well as population-level differences in disease prevalence and etiology, there is unlikely to be a one-size-fits-all therapeutic strategy for treating fibrosis. In vitro models can be used to study patient-specific responses to different treatments, which can help create personalized medicine approaches for patients affected by fibrosis.
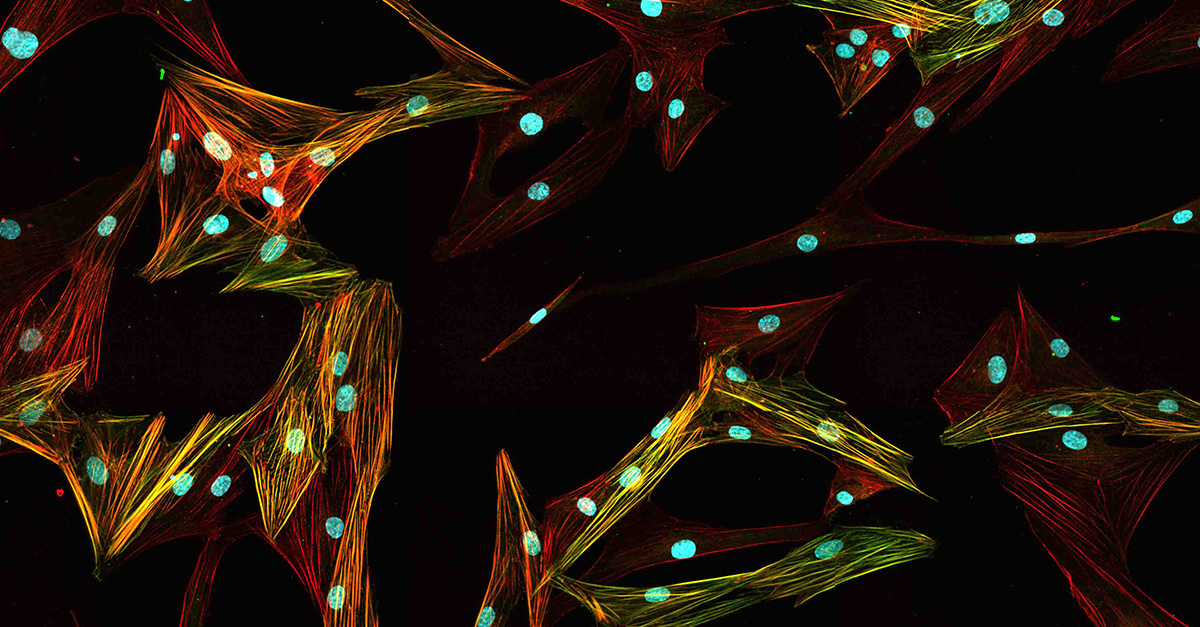
Primary normal human lung fibroblasts stained with Hoechst, anti-alpha-SMA antibody, and phalloidin.
In vitro models are typically amenable to automation and scale-up. This allows researchers to evaluate a large number of anti-fibrotic compounds and treatment strategies in parallel within a relatively short time frame. The compatibility of in vitro models with high-throughput screening accelerates the drug discovery process by rapidly identifying promising drug candidates for further investigation.
Importantly, fibrosis studies using in vitro models can be a cost-effective alternative to in vivo studies. Animal models of fibrosis are notoriously time- and labor-intensive, requiring specialized facilities and expertise to maintain, and the presentation of fibrosis in animal models is not fully representative of human biology. Additionally, ethical concerns limit how such models can be utilized in research studies. In a move toward reducing the use of animals in research, the U.S. Food and Drug Administration recently removed the requirement for drugs to be tested in animals before being considered for human clinical trials. In this way, in vitro models provide an affordable means to obtain useful preclinical data while circumventing ethical issues in research.
ADVANCEMENTS IN IN VITRO FIBROSIS MODELS
Recent cell culture advancements have improved the relevance and reliability of in vitro fibrosis models, increasing their utility in fibrosis research. One major advancement is the development of three-dimensional (3D) in vitro models that better mimic the complex architectures, matrix environments, and intercellular interactions of in vivo tissues than traditional two-dimensional (2D) in vitro models. While 2D culture causes fibroblasts to rapidly lose their native cellular characteristics, culturing fibroblasts and other cell types in 3D within physiologically relevant matrix environments promotes the formation of tissue-like structures that serve as more accurate and controlled fibrotic models.
Dr. Marie Besnier, Head of Life Science at Inventia Life Science, attests to the value of 3D in vitro models: “Before 3D models, studying fibroblasts was a race against time, as the cells would lose their essential natural characteristics very quickly. 3D models enable us to preserve their function for an extended amount of time, which allows us to properly study them.”
Another recent advancement is the ability to culture primary human cells in in vitro models. Using cells obtained from patients affected by fibrosis, researchers can study patient-specific responses to treatments and identify fibrosis-related biomarkers to provide diagnostic and prognostic guidance. In addition, the integration of more advanced imaging techniques and molecular analysis tools with 3D in vitro models has revolutionized in vitro assays. Live-cell imaging allows researchers to visualize and track cellular processes in real time, revealing the dynamic nature of fibrosis development. Molecular analysis in the form of gene expression profiling and proteomics enables researchers to study the molecular changes associated with fibrosis and identify potential therapeutic targets.
Overall, current in vitro models better mimic the complexity of fibrotic tissues to investigate therapeutic interventions, study patient-specific responses, and improve our understanding of fibrosis.
APPLICATIONS OF IN VITRO FIBROSIS MODELS IN DRUG DEVELOPMENT
In drug development, in vitro models serve as the foundation for the initial screening of candidate anti-fibrotic drugs and interventions, with the aim of identifying promising candidates for further investigation. As in vitro models are highly scalable, numerous drugs can be assessed simultaneously in automated workflows that reduce labor costs and save time. Additionally, the flexibility that in vitro models offer enables the design of experiments to target specific fibrotic readouts or markers of interest in drug discovery. For example, to develop a drug that targets collagen deposition in fibrosis, researchers can model collagen deposition in vitro by adjusting the matrix composition, stiffness, cell ratios, and time points, to yield robust collagen readouts for testing. This ability to control and manipulate biological phenomena using in vitro models allows researchers to perform mechanistic studies, including the study of how drug candidates interact with specific cellular targets or signaling pathways involved in fibrosis progression. This can guide drug design and help overcome potential resistance mechanisms.
In addition to drug discovery and development, in vitro fibrosis models can be used in toxicity testing. Researchers can assess the safety profiles of drug candidates, identifying possible adverse effects or toxicity concerns before advancing to preclinical and clinical studies. Increasing the physiological relevance of the in vitro model allows for more accurate toxicity assessments.
CHALLENGES AND FUTURE DIRECTIONS IN FIBROSIS RESEARCH
Fibrosis research is a rapidly evolving field. As our understanding of fibrosis deepens, in vitro models are increasingly mimicking the complexity of fibrotic tissues via the incorporation of multiple cell types, extracellular matrix components, and fluid flow. For this reason, in vitro models have moved into 3D and microfluidic formats to provide more accurate representations of fibrosis.
To better understand fibrosis at the molecular level, researchers are using multi-omic approaches. By combining genomics, transcriptomics, proteomics, and metabolomics data, researchers can capture molecular changes associated with the disease at a systemic level. This holistic approach will facilitate the identification of novel biomarkers, therapeutic targets, and personalized treatment strategies.
The translation of in vitro data to in vivo models and clinical settings remains a significant challenge in fibrosis research. While in vitro models offer controlled and reproducible conditions, they may not fully capture the complexity of fibrosis and the interactions between fibroblasts and other cell types within the in vivo tissue microenvironment. Collaborative efforts between researchers, clinicians, and industry partners will be essential for bridging the gap between in vitro and in vivo studies to ultimately transform promising preclinical findings into effective therapeutic interventions for fibrosis.
In summary, recent advancements in in vitro fibrosis models are facilitating our understanding of fibrosis and rapidly accelerating fibrosis research. Increasing in vitro model complexity and validating new fibrosis biomarkers will be crucial for developing effective therapies for fibrosis and improving patient outcomes. Key future directions for the field of fibrosis research include the development of more sophisticated in vitro models, the utilization of multi-omics approaches, and the translation of in vitro findings to in vivo and clinical settings.
ADDITIONAL ARTICLES TO EXPLORE
Zhang, C., Meyer, S., et al. (2023). A human liver organoid screening platform for DILI risk prediction. Journal of Hepatology, 78(5), 1-9. https://www.journal-of-hepatology.eu/article/S0168-8278(23)00072-7/fulltext
Vazquez-Armendariz, A., Barroso, M., et al. (2022). 3D in vitro models: Novel insights into idiopathic pulmonary fibrosis pathophysiology and drug screening. Cells, 11(9), 1526. https://www.mdpi.com/2073-4409/11/9/1526

APPLICATION AND TECHNICAL NOTES
Studying Fibroblast Activation and Production of Extracellular Matrix Proteins with RASTRUM™ 3D Cell Models
Download NowThis application note outlines the creation of 3D cell models using human lung fibroblasts and liver stellate cells in RASTRUM™ Matrix, showcasing their viability and applicability in diverse areas, including phenotypic drug screening.