OVERVIEW
In vitro cellular models are a centerpiece for understanding, modifying, and quantifying the complexity of gene delivery. Induced pluripotent stem cells (iPSC) provide the ability to interrogate biologically relevant cell types in vitro. These cell types are compatible with
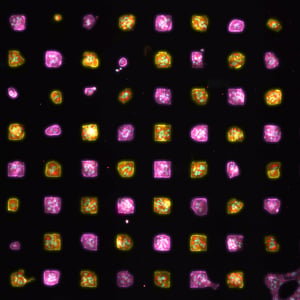
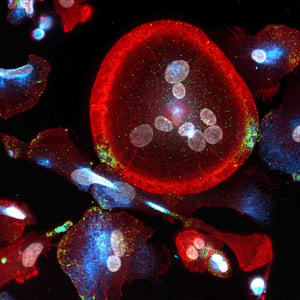
various gene targeting approaches, particularly adeno-associated viral (AAV) vectors. Defining AAV transduction permissibility across different iPSC-derived cell types is a necessity. Quantifying the effects of viral vector serotype, multiplicity of infection (MOI), promoter, media, and assay timing in a human-focused model system is possible with iPSC-derived cells.
These specialized cell types offer a major advancement for AAV studies of gene delivery.
RELATED RESOURCES
CONTACT US
Let's discuss how we can help with your next study.
Evaluating Transduction Efficiencies of AAV Vectors into Human iPSC-derived Cell Types
Overview
The differentiation of induced pluripotent stem cells (iPSC) into specialized cell types of the human body represents a major advancement for the development of biologically relevant in vitro models. These cellular systems enable “disease-in-a-dish” studies and are compatible with various gene targeting approaches, including adeno-associated viral (AAV) vectors, to directly correct the genetic mutation. However, the permissibility of AAV transduction across different iPSC-derived cell types needs to be defined. This requires the implementation of a sensitive and versatile technology to monitor transduction efficiencies and quantify the impact of various factors, including viral vector serotype, multiplicity of infection (MOI), choice of promoter, transduction media, and/or timing of transduction.





