Adipose tissue (a.k.a., fat), plays a crucial role in energy storage and metabolism. While people commonly associate fat with weight gain, it’s not that simple. The two main types of adipose tissue are white adipose tissue (WAT) and brown adipose tissue (BAT), and the balance of these and their functions is key to governing metabolic health. Differences in this balance help explain the differences in metabolism between individuals and between species. Here, we review the differences between WAT and BAT and, in honor of the National Park Service’s Fat Bear Week, investigate bears’ amazing abilities to store up on fat for hibernation in the winter.
TABLE OF CONTENTS
What Can We Learn From Understanding Bear Metabolism?
WHITE ADIPOSE TISSUE (WAT)
WAT is the most abundant type of fat in the human body and is comprised primarily of adipocytes. Its primary functions are energy storage, insulation, and protection/cushioning of vital organs. During periods of energy surplus, precursor cells called preadipocytes undergo adipogenesis, a process in which they differentiate into mature adipocytes. This process is tightly regulated by several factors, including genetics, hormones, and environmental influences.
In adipocytes, energy is stored as triglycerides, which appear as fat droplets in the cells. When the body requires energy, stored triglycerides undergo lipolysis, a process where they are broken down into fatty acids and glycerol. These released fatty acids are then transported through the bloodstream to other tissues to meet energy demands. Fatty acids are transported to the mitochondria within cells, where they undergo a process called beta-oxidation, ultimately generating adenosine triphosphate (ATP), the energy currency of the body. When energy demands increase, such as during exercise or fasting, the body taps into these fat stores to provide the necessary fuel.
WAT also secretes hormones and signaling molecules called adipokines, which are key to regulating appetite, metabolism, inflammation, and insulin sensitivity. Over-accumulation of WAT leads to obesity and increased risk of associated metabolic diseases, such as type 2 diabetes, cardiovascular disease, and some cancers. Dysregulation of adipokine secretion contributes to the development of these conditions, and researchers are investigating strategies to modulate WAT function in the development of therapeutics for preventing or treating metabolic diseases.
BROWN ADIPOSE TISSUE (BAT)
BAT derives its brown color from the high density of mitochondria in its cells and its rich blood supply. The key player in BAT’s thermogenic function is a mitochondrial protein called uncoupling protein 1 (UCP1), which uncouples the process of oxidative phosphorylation from ATP production. This allows protons to leak across the inner mitochondrial membrane to generate heat instead of producing ATP. When the body is exposed to cold temperatures or certain hormones, BAT activates thermogenesis, increasing the metabolic rate and burning stored fat to produce heat and maintain the body’s core temperature.
Given its ability to increase energy expenditure, BAT activation and WAT-to-BAT conversion have garnered significant interest as avenues for developing weight-management interventions. Developing therapies that increase BAT activity could help people burn more calories and aid in weight-loss efforts to combat the obesity epidemic.
BAT's role in glucose homeostasis and lipid metabolism suggests that targeting BAT could also be effective in treating metabolic disorders, such as type 2 diabetes and dyslipidemia. By improving insulin sensitivity and lipid profiles, BAT activation could offer new treatment opportunities for these conditions. Several compounds, such as β3-adrenergic receptor agonists, have been shown to increase BAT thermogenesis, but further research is needed to ensure their safety and effectiveness in patients.
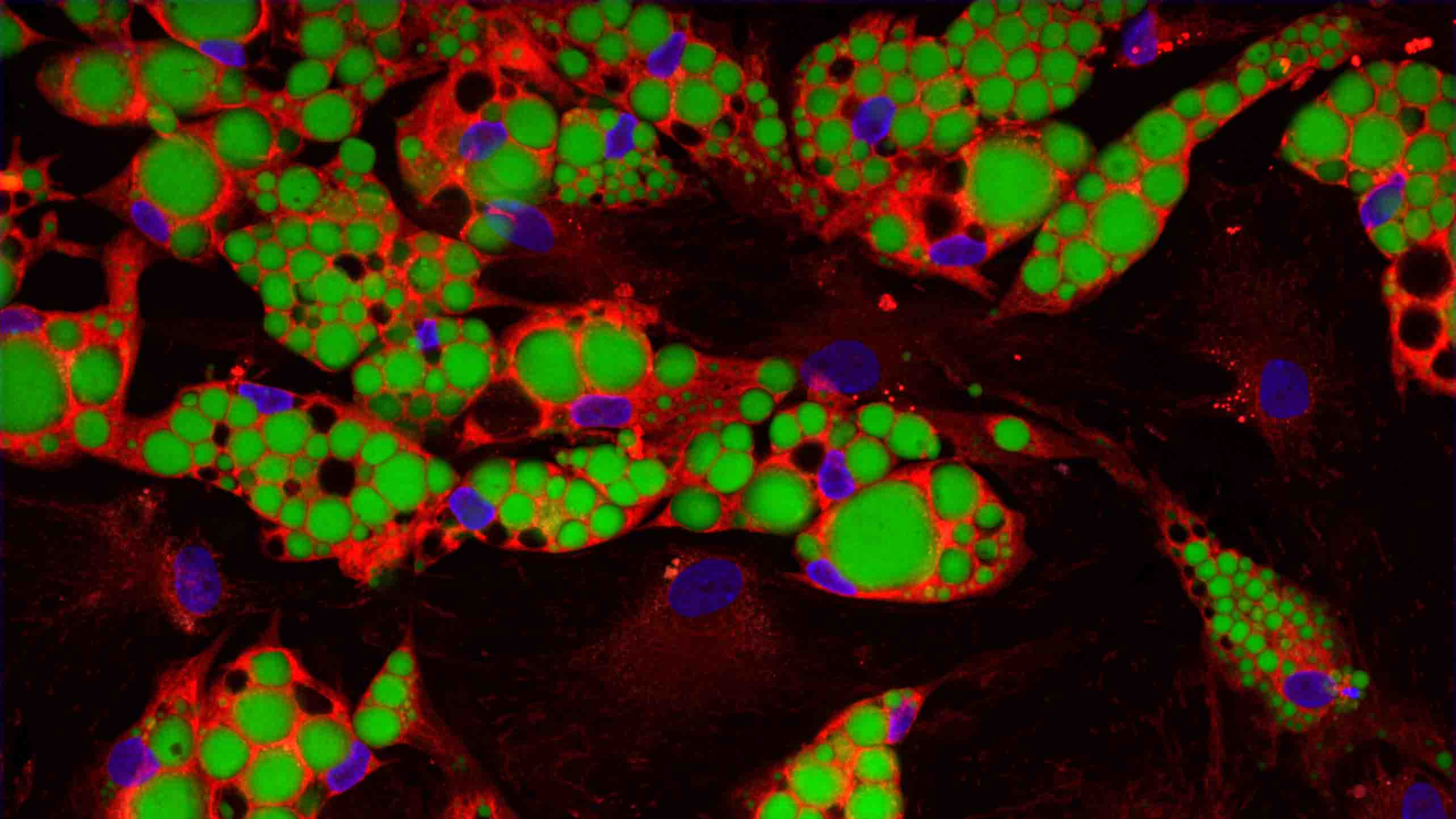
Adipocytes stained with Hoechst, BODIPY, MitoTracker Deep Red FM
BEAR HIBERNATION METABOLISM
Bear hibernation is an amazing phenomenon that allows them to survive for months without eating, drinking, or excreting waste. Hibernation relies on WAT as the primary energy source, so as bears prepare for winter, they undergo a period called hyperphagia, during which they consume up to 20,000 calories each day to build up their fat reserves. During hyperphagia, an increased production of insulin-like growth factor 1 stimulates the proliferation of preadipocytes and subsequent adipogenesis, which is under the control of various hormones, including leptin, insulin, and glucagon-like peptide.
During hibernation, bears experience extreme reductions in metabolic activity, body temperature, heart rate, and respiration. This remarkable adaptation enables them to preserve energy during periods of scarce food availability, such as winter.
Unlike in human tissues, bears' adipose tissue exhibits remarkable resistance to the negative effects of prolonged immobility and fasting. Bears' adipose tissue remains insulin-sensitive during hibernation, so they maintain stable blood-glucose levels and preserve their fat stores. Additionally, the fat stored in bears' adipose tissue in the winter has higher proportions of polyunsaturated fatty acids than in the summer months. This change in fat composition reflects the shift in fatty-acid mobilization that occurs in bears when they hibernate.
Also unlike humans, bears possess a higher ratio of BAT to WAT. This is because every 1-2 weeks during hibernation, stored lipids are consumed at high rates in BAT to generate heat through lipolysis, and these whole-body, re-warming events are called interbout arousals (IBAs). IBAs allow bears to maintain their core body temperature despite reduced metabolic activity. This unique form of fat metabolism is known as "non-shivering thermogenesis.”
WHAT CAN WE LEARN FROM UNDERSTANDING BEAR METABOLISM?
Adipogenesis is a complex process that varies between humans and bears due to their unique physiological adaptations. While the human metabolic system is designed to support an active lifestyle, bears’ hibernation metabolism focuses on energy conservation. Understanding the similarities and differences in adipogenesis between these species not only enhances our knowledge of biology, but also provides insights into potential applications in human health.
By studying these complex and distinct metabolic processes, such as the atrophy-resistant and insulin-sensitive qualities of bear adipose tissue, scientists may uncover new ways to develop therapies for metabolic disorders. The unique adaptations seen in bears during hibernation, including the ability to tolerate high levels of circulating fatty acids and their unique, non-shivering thermogenesis, contribute to their survival during extended periods of fasting, and can clue scientists into strategies for energy conservation for humans in extreme conditions.
Ongoing research in this field may lead to innovative approaches for manipulating adipose tissue function, ultimately benefiting human health. As scientists continue to unravel the mysteries of fat metabolism, we can look forward to advancements in our understanding of adipose tissue and its potential applications in combating obesity and related metabolic conditions.
REFERENCES:
- Ballinger, M.A., Andrews, M.T. (2018). Nature’s fat-burning machine: brown adipose tissue in a hibernating mammal. J Exp Biol., 221 (Suppl 1).
- Carey, H. V., Andrews, M. T., & Martin, S. L. (2003). Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiological reviews, 83(4), 1153-1181.
- Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):143-149.
- Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11(4):253-256.
- Kajimura S. and Saito M. (2014). A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annual Review of Physiology, 76, 225-249.
- Nelson, R. A., & Folk, G. E. (2000). Hyperphagia, food consumption and energy utilization in bears. In International Conference on Bear Research and Management (Vol. 8, pp. 177-187).
- Rosenwald M. and Wolfrum C. (2014). The origin and definition of brite versus white and classical brown adipocytes. Adipocyte, 3(1), 4-9.
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500-1508.
- Wu J. et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell, 150(2), 366-376.