In recent years, there have been groundbreaking developments in the field of oncology research, particularly in the realm of cell-based therapies. One such technique that has garnered significant attention is Chimeric Antigen Receptor T-cell (CAR-T) therapy. This revolutionary method of treatment holds immense promise in the fight against cancer, offering new avenues for personalized medicine. In this blog post, we will delve into the intricacies of CAR-T therapy and explore its potential applications in the field of oncology research.
TABLE OF CONTENTS
Clinical Applications of CAR-T Therapy
Improving the Efficacy of CAR-T Therapy
Managing CAR-T Therapy Toxicity
The Role of High-content Imaging in CAR-T Therapy Research
UNDERSTANDING CAR-T THERAPY
CAR-T therapy is a cutting-edge immunotherapy technique that harnesses the power of a patient's own immune system to find and destroy cancer cells. It involves genetically modifying T-cells to express recognition proteins called chimeric antigen receptors (CARs) on their surface and then introducing these modified, CAR-T cells into the patient.
CARs recognize and bind to specific antigens that are highly expressed by cancer cells but are either not expressed or expressed at low levels by normal cells. This targeted approach minimizes damage to healthy cells, distinguishing CAR-T therapy from traditional, cancer treatments, such as chemotherapy or radiation, which harm healthy cells and lead to severe, even deadly, side effects in the patient.
CLINICAL APPLICATIONS OF CAR-T THERAPY
CAR-T therapy has had success in fighting hematological malignancies, including acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and some lymphomas. Clinical trials have demonstrated high response rates and even complete remission. Conversely, CAR-T therapy has had much less success with solid tumors, largely due to the heterogeneity of tumors. Other contributors to this failure include shallow tumor penetration by the CAR-T cells and the inability of CAR-T cells to survive in the immunosuppressive tumor microenvironment. Active research focuses on how to improve CAR-T cell trafficking and prolong their survival in tumors, so they can access and act upon their targets.
IMPROVING THE EFFICACY OF CAR-T THERAPY
As CAR-T therapy continues to revolutionize the field of oncology, researchers are tirelessly working to enhance its efficacy. One area of focus is optimizing the design of CARs to improve their binding affinity and specificity for cancer cells. By fine-tuning the structure of the CAR molecule, scientists aim to ensure a more robust and targeted immune response.
In addition to CAR design, improving T-cell expansion and persistence is a critical aspect of enhancing CAR-T therapy efficacy. Researchers are exploring various methods to enhance the expansion and longevity of CAR-T cells within the patient's body, such as optimizing the culture conditions for T-cells during the manufacturing process and incorporating genetic modifications that promote their survival and proliferation.
Another significant challenge in CAR-T therapy is overcoming the immune-escape mechanisms employed by cancer cells. Cancer cells can develop strategies to evade recognition and destruction by CAR-T cells, leading to treatment resistance and disease progression. To counter this challenge, researchers are developing innovative approaches to enhance CAR-T cell function and responsiveness, including incorporating co-stimulatory molecules into the CAR design or utilizing combination therapies that target multiple, cancer-specific antigens simultaneously.
MANAGING CAR-T THERAPY TOXICITY
While CAR-T therapy has shown remarkable success in treating certain types of cancer, it has also induced adverse events that require careful management. Cytokine release syndrome (CRS) and neurotoxicity are two common toxicities associated with CAR-T therapy.
Cytokine release syndrome occurs when CAR-T cells are activated and release large amounts of pro-inflammatory cytokines, leading to systemic inflammation. This results in flu-like symptoms, fever, and, in severe cases, organ dysfunction. To manage CRS, researchers are developing strategies to mitigate its severity by using immunomodulatory drugs or cytokine-blocking agents. Additionally, efforts are underway to identify biomarkers that can predict the likelihood and severity of CRS, allowing for early intervention and tailored treatment.
Neurotoxicity, another potential adverse event, manifests as confusion, seizures, or other neurological symptoms. The exact cause of neurotoxicity in CAR-T therapy is not fully understood, but it is believed to involve the activation of immune cells in the central nervous system. Researchers are actively investigating the mechanisms underlying neurotoxicity and developing strategies to prevent or manage its occurrence. Some strategies include optimizing CAR design to minimize off-target effects and exploring the use of anti-inflammatory or neuroprotective agents.
Overall, the management of toxicities associated with CAR-T therapy is a critical aspect of ensuring patient safety and improving outcomes. Ongoing research and advancements in this area will continue to refine the therapeutic approach, making CAR-T therapy an even more viable and effective treatment option for cancer patients.
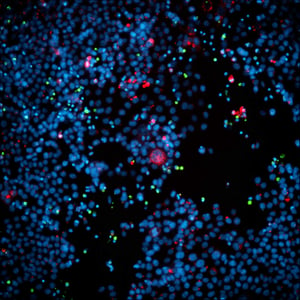
%20LR%202.jpg?width=500&height=520&name=MicrosoftTeams-image%20(1)%20LR%202.jpg)
A549 spheroid co-cultured with CAR-T cells for 48 hours, labeled for nuclei (blue), A549 cells (CellTracker Green), and CAR-T cells (CellTracier Red).
THE ROLE OF HIGH-CONTENT IMAGING IN CAR-T THERAPY RESEARCH
High-content imaging allows researchers to visualize and quantitatively analyze the interactions between CAR-T cells and cancer cells at a microscopic level in a scalable format for screening. Assays can be run in 2D or 3D formats and can use any type of cell, from standard, immortalized cell lines to patient-derived tissues.
By using high-resolution imaging techniques, scientists can track the location and behavior of CAR-T cells within patient-derived, solid-tumor fragments, providing valuable insights into CAR-T cell design and killing efficacy. Additionally, high-content imaging enables the identification of potential biomarkers or signaling pathways that can be leveraged to enhance CAR-T cell function, such as by designing better CARs. These in vitro assay designs can be highly useful for evaluating CAR-T specificity for cancer cells vs. healthy cells, offering an assessment of toxicity as well.
High-content imaging empowers researchers to optimize CAR-T therapy by providing a visual understanding of its mechanism of action and guiding the development of strategies to overcome the challenges faced in treating solid tumors. With the continuous advancements in imaging technology and assay techniques, the future of CAR-T research looks promising.
FUTURE DIRECTIONS
Researchers are devising strategies to overcome the challenges associated with CAR-T therapy to enhance its promise as an effective cancer treatment. For instance, scientists are actively investigating the synergistic effects of combining CAR-T therapy with other treatment modalities, such as immune checkpoint inhibitors, small-molecule inhibitors, or other cell-based therapies. These approaches aim to enhance the overall anti-cancer response and reduce the risk of relapse. Furthermore, great efforts are being made to overcome the challenges posed by solid tumors from both the tumor and CAR-T cell sides: Better understanding the heterogeneous intricacies of tumor microenvironments will inform researchers about how to overcome them. Improving the resilience and trafficking of CAR-T cells will result in improved target access and tumor killing.
CAR-T therapy and cell-based oncology research represent a paradigm shift in cancer treatment. With its ability to harness the power of the immune system to target cancer cells specifically, this innovative technique can revolutionize oncology care. As research in this field continues to progress, we can look forward to advancements that will expand the scope of CAR-T therapy, enabling it to benefit a broader range of patients and solidifying its place as a promising frontier in the fight against cancer.
REFERENCES:
- Sadelain, M., Rivière, I., & Riddell, S. (2017). Therapeutic T cell engineering. Nature, 545(7655), 423-431.
- June CH, Sadelain M. (2018). Chimeric Antigen Receptor Therapy. N Engl J Med, 379(1), 64-73.
- Grupp SA, Kalos M, Barrett D, et al. (2013). Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med. 368(16), 1509-1518.