In recent years, advancements in understanding and engineering gene-delivery vectors have brought gene therapy to the forefront of medical research for treating genetic diseases at their source. Among these numerous vectors, adeno-associated viruses (AAVs) have emerged as one of the most promising tools for delivering genetic material to target cells, with several new therapies receiving FDA approval just this year. In this post, we delve into the significance of AAVs as gene-therapy vectors and practical considerations for their use.
TABLE OF CONTENTS
Challenges in Developing AAV-based Gene Therapy
WHAT ARE AAVs?
Originally discovered as an incidental presence in adenovirus preparations, AAVs are deceptively simple entities composed of a protein shell guarding a small, single-stranded DNA genome. Within this structure are various molecular intricacies, featuring three critical genes: Rep (Replication), Cap (Capsid), and Aap (Assembly-activating protein). These genes coordinate the AAV’s ability to replicate, package genetic cargo, and interact with host cells.
WHY USE AAVs?
Although there are many vectors that have been and are being tested for use in gene therapy, AAVs offer a unique set of advantages. For one, they are dependent on helper viruses (like adenovirus) to replicate in a host, limiting their pathogenicity. Although they do not replicate and their genomes do not generally integrate into the host cell genome, they are capable of long-term gene expression, allowing for sustained, therapeutic-protein production. This characteristic is particularly advantageous for genetic diseases, where ongoing gene expression is necessary for lasting therapeutic effects.
Another advantage of using AAVs is that their infections are asymptomatic, with low immunogenicity compared to other vector types. Most of us are naturally infected with a variety of AAVs throughout our lifetime – infections which do not cause an identifiable, associated disease and which can last a lifetime without causing any subsequent issues. Extensive research and clinical trials have shown that AAV vectors are well-tolerated by patients, with minimal side effects and low risk of insertional mutagenesis.
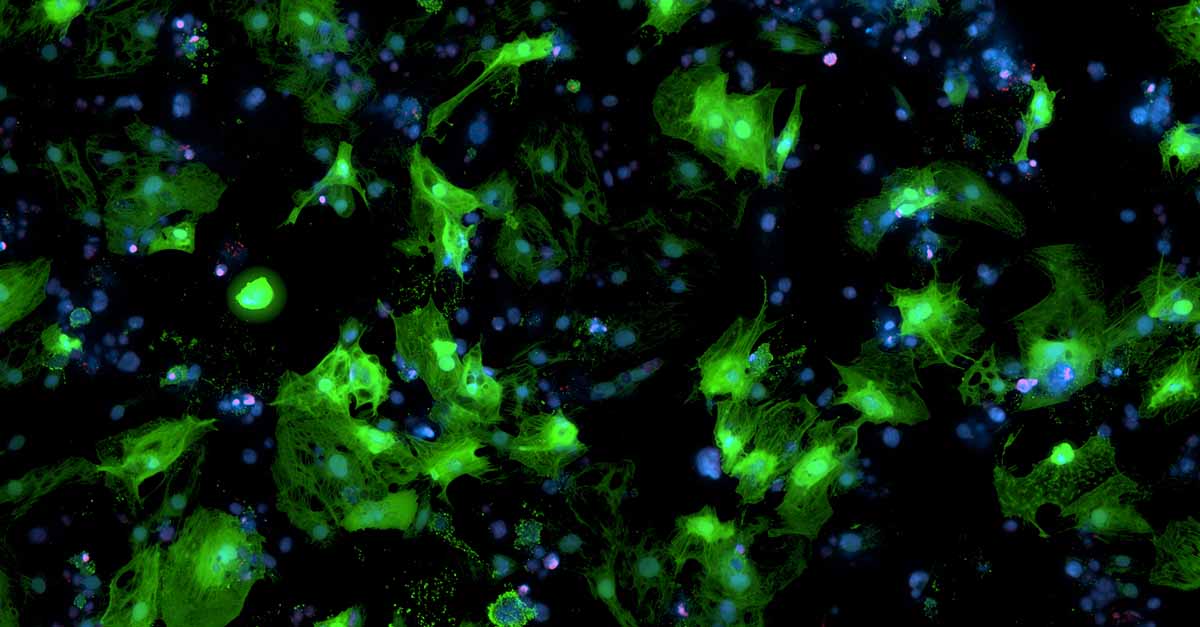
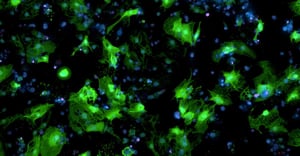
Human, iPSC-derived cardiomyocytes transduced with AAV carrying a GFP gene.
AAV SEROTYPES
There are many types of AAVs - known as serotypes - which occur naturally. AAV serotypes differ in their tissue tropism (i.e., their ability to infect a specific cell or tissue type) due primarily to their varied surface antigens, as determined by the makeup of their capsid protein. Each serotype contains different cap genes, resulting in the expression of various forms of structural proteins VP1, VP2, and VP3. These proteins form the AAV capsid and have varying levels of homology, but they all adopt a similar geometrical structure to form the viral particle. As the capsid directly interacts with host cells, variations in its structure confer differential abilities to bind cellular receptors. On a macromolecular scale, this results in specificities of some serotypes for particular cell or tissue types because different types of cells express different receptors or different levels of certain receptors. This physical diversity of AAV serotypes introduces complexity and choice into the preclinical landscape.
Scientists are now improving upon the innate ability of AAV serotypes to target specific tissues in order to develop gene therapies. Using various biochemical techniques, novel capsids can be rationally designed, and combinatorial libraries of capsids can be created and screened against panels of cell types to select for those that show the greatest specificity and, theoretically, the greatest chances of clinical success. For instance, when developing a drug for retinal disease, you would want the vast majority of AAVs to be taken up by retinal cells. Unfortunately, the tissue tropism of AAV serotypes is not clear-cut. For instance, AAV2 exhibits broad tropism for many tissues, including retinal cells; however it also targets liver, kidney, and pancreas cells. Its tropism is further dependent on the route of administration. This can make tissue-specific targeting difficult, and the ability to develop and characterize new capsids, whether through library screening or rational design, can improve upon some limitations of naturally derived AAV serotypes.
By combining cutting-edge biochemical techniques, improved disease models, and greater screening technologies, researchers can better understand AAV-host cell interactions and engineer greater precision into AAV vectors to develop safe and effective gene therapies.
CHALLENGES IN DEVELOPING AAV-BASED GENE THERAPY
Despite the FDA approval of multiple AAV-based gene therapies recently, the continued advancement of AAV as a safe and efficient vector faces notable challenges.
At the production level, researchers commonly encounter low vector concentrations and high levels of empty or partially filled capsids. This low fill efficiency is problematic because large numbers of AAV capsids must be injected to reach a certain concentration of functional vectors, which can elicit a strong immune response that can be fatal to the patient. Two commonly used approaches to remove the empty and partial capsids are stratification of the empty and full capsids through analytical ultracentrifugation or isoelectric focusing. New techniques for both packaging the vectors and separating the empty/partial capsids are under development to increase vector-production efficiency.
Another challenge that results in high dose administration is the broad tropism of AAV serotypes. Large amounts of non-specific vectors must be injected into a patient to reach effective doses in the cell or tissue type of interest because the vectors are taken up by untargeted tissues or accumulate in the liver. This phenomenon can lead to strong immune responses or liver failure in the patient. Scientists are tackling this challenge by engineering capsids that confer greater specificity for target tissues and by developing in vitro models that faithfully mirror human biology.
Engineering new capsids can also address the issue of immunogenicity of AAV administration. Researchers are designing new capsid proteins that may be able to evade the immune system’s surveillance or at least elicit a weaker response. The successful execution of this strategy requires a deep understanding of the immune system – a challenging feat, as each person’s immunological biology is unique and ever-evolving.
Despite these challenges, the increasing number of FDA approvals of AAV-based therapies and the incredible expansion of AAV research over the last decade foreshadow a promising future for treating a variety of recalcitrant diseases. This resurgence of interest in and research around AAVs has, and will continue to, not only afford new treatments for genetic diseases, but also deepen understandings of the genetic intricacies of diseases to continue propelling the development of new therapeutic discoveries.
REFERENCES:
-Au, Hau Kiu Edna, Isalan, Mark, Mielcarek, Michal (2022). Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Front. Med., 8(809118).
-Naso, Michael F., Tomkowicz, Brian, Perry, William L., Strohl, William R. (2017). Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs, 31(4), 317-334.
-Pupo, Amaury, Fernandez, Audry, Low, Siew Hui, Francois, Achille, Suarez-Amaran, Lester, Samulski, Richard Jude (2022). AAV vectors: The Rubik’s cube of human gene therapy. Mol Ther. 30(12): 3515-3541.
-Verdera, Helena Costa, Kuranda, Klaudia, Mingozzi, Federico (2020). AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol Ther. 28(3):723-746.
-Wang, D. Tai, Phillip W.L., Gao, Guangping (2019). Adeno-associated virus vector as a platform for gene therapy delivery. Nature Reviews Drug Discovery, 18, 358-378.